A study says that a combination of Covaxin and Covishield vaccines has elicited better safety and immunogenicity results
Our Bureau
London/New Delhi
United Kingdom government on Wednesday in its revised travel advisory said that Covishield is qualified as an approved vaccine. Earlier, the UK government did not accept people vaccinated with Covishield and considered them as unvaccinated. The need for them to go through 10-day quarantine was criticized in India. The new travel advisory by the UK stated that the four listed vaccines, such as AstraZeneca Covishield, AstraZeneca Vaxzevria and Moderna Takeda, qualify as approved vaccines.
But there is still confusion over the United Kingdom’s vaccine recognition process for Indian travellers. Even though UK’s new travel rules state that AstraZeneca Covishield is among vaccines that qualify as approved, India is not yet on the list of 17 countries mentioned. The ‘changes to international travel rules’ state that formulations of the four listed vaccines, such as AstraZeneca Covishield, AstraZeneca Vaxzevria and Moderna Takeda, qualify as approved vaccines.
Earlier, India had said on Tuesday that it will be within its rights to take reciprocal measures against UK’s “discriminatory” move to recognize AstraZeneca Covid-19 vaccine but not Covishield if the issue is not satisfactorily resolved.
Foreign Secretary Harsh Vardhan Shringla told media persons on Tuesday that External Affairs Minister S Jaishankar had raised the issue strongly with the UK.
“Have raised the discriminatory nature of UK vaccine recognition for AstraZeneca but not Covishield. Discussions on, but if they do not satisfy us, we would be well within our rights to take reciprocal action,” Shringla had said.
“Non-recognition of Covishield is a discriminating policy and impacts Indian citizens travelling to the UK. I am told that certain assurances have been given that this issue will be resolved,” he added.
Amid concerns in India about UK’s new travel rules, British High Commissioner to India Alex Ellis on Wednesday said that Covishield is not a problem and detailed technical discussions are being held regarding certification of COVID-19 vaccine with the builders of CoWIN app and NHS app.
“We’re clear Covishield is not a problem. The UK is open to travel and we’re already seeing a lot of people going from India to the UK, be it tourists, business people or students. Over 62,500 student visas have been issued in the year ending June 2021, which is an increase of almost 30 per cent as compared to the previous year,” Ellis said in a statement. “We have been having detailed technical discussions regarding certification, with the builders of the CoWIN app and the NHS app, about both apps. They’re happening at a rapid pace, to ensure that both countries mutually recognize the vaccine certificates issued by each other,” he added.
CoWIN is the technological backbone driving vaccination in India and encompasses all constituents of the vaccination process. There has been confusion over UK’s vaccine recognition process for Indian travellers. Even though these rules state that AstraZeneca Covishield is among vaccines that qualify as approved, India is not yet on the list of 17 countries mentioned.
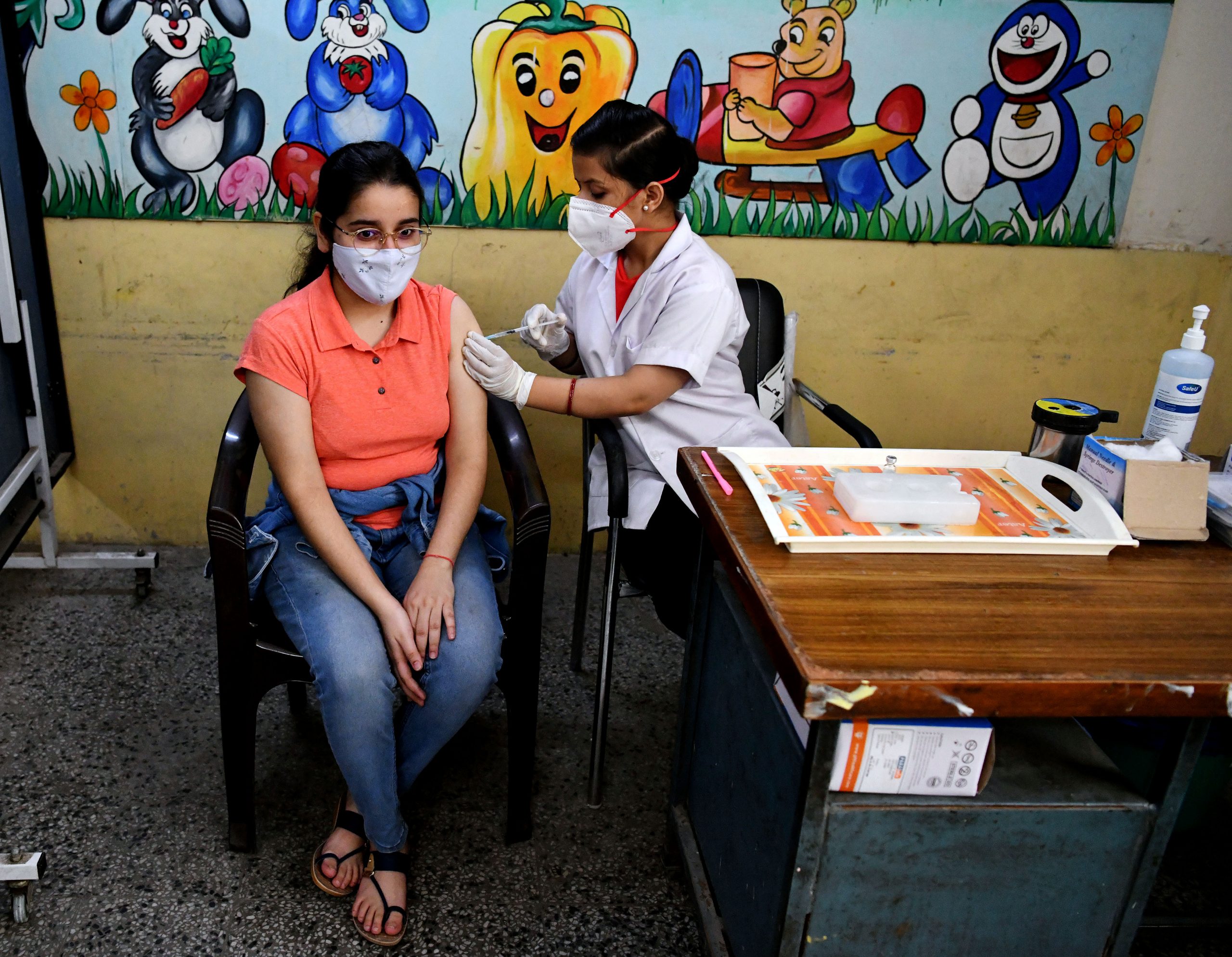
Meanwhile, the Indian Council of Medical Research (ICMR) has revealed in its study that the combination of Covaxin and Covishield vaccines, the two main vaccines of the COVID-19 vaccination programme, conducted on 18 people, has elicited better safety and immunogenicity results. The nationwide vaccination program had entered into 4th month when the event of mixed dosing raised considerable anxiety in the public domain with the potential to contributing to vaccine hesitancy. ICMR conducted the current investigation against that backdrop.
As per Dr Samiran Panda, Head of Epidemiology and Communicable Diseases, ICMR, “it was like a natural experiment when second time these individuals inoculated with different doses inadvertently.”
He informed that ICMR decided to conduct the study so that people would not get anxiety or vaccine hesitancy. “We went to the place and collected samples of individuals,” said Dr Panda.
A total of 18 participants were in the heterologous group. However, two participants were unwilling and were excluded. Among them, 11 were male, and seven were female with a median age of 62 years. “A comorbid condition (hypertension) was reported in one (5.5 per cent) individual. In both the homologous groups, 40 individuals were included,” the study said.
The findings of the study suggest that immunization with a combination of an adenovirus vector platform-based vaccine followed by an inactivated whole virus vaccine was not only safe but also elicited better immunogenicity.