Very small number of doses coming from the US. Chaotic vaccination plan. Pfizer and Moderna demanding indemnity in India. Everything that could go wrong with India’s vaccine efforts has gone wrong. What is the government doing to correct the course?
Our Bureau
New Delhi
In a huge development on Thursday, US Vice President Kamala Harris spoke to Prime Minister Narendra Modi over the Covid-19 vaccine strategy. The phone call was initiated by Kamala Harris after US President Joe Biden announced his plan to share some 25 million of a planned 80 million Covid-19 vaccines with the rest of the world. The United States will donate nearly 19 million doses of its Covid-19 vaccine through the COVAX facility, he said in a statement.
Earlier on Thursday, President Joe Biden announced the first details of the U.S. sending 80 million COVID-19 vaccines overseas with the aim of “ending the pandemic globally.” At least 75 percent of these doses—nearly 19 million—will be shared through COVAX, including approximately 6 million doses for Latin America and the Caribbean, approximately 7 million for South and Southeast Asia, and approximately 5 million for Africa. “The remaining doses, just over 6 million, will be shared directly with countries experiencing surges, those in crisis, and other partners and neighbors, including Canada, Mexico, India, and the Republic of Korea,” Biden said.
It is not clear how many doses India will get. It is also not clear if India will get any doses under the COVAX scheme as it was supposed to be the biggest contributor to the WHO program. Under the US plan, India may get under 6 million doses, which is a very small number, considering India’s population and the magnitude of crisis.
The announcement came as India gets desperate for vaccines. The vaccination plan is moving slowly and in a chaotic manner. So much so that even the Supreme Court has taken the Modi government to task. Underlining that “our Constitution does not envisage courts to be silent spectators when constitutional rights of citizens are infringed by Executive policies”, the Supreme Court said Tuesday that the Centre’s policy of arranging free Covid-19 vaccine jabs for the 45-plus age category, Health Care Workers (HCW) and Front Line Workers (FLW) while asking the 18-44 age group to pay for the vaccination by state and Union territory governments and private hospitals was “prima facie arbitrary and irrational”.
Directing the Centre to “undertake a fresh review of its vaccination policy addressing the concerns raised”, the bench of Justices D Y Chandrachud, L Nageswara Rao and S Ravindra Bhat — the order in the suo motu matter of Covid-19 management heard May 31 was uploaded Tuesday — sought detailed information in the form of an affidavit in two weeks.
The bench said: “Unlike the prior policy, the Liberalized Vaccination Policy does not prioritize persons with co-morbidities and other diseases, persons with disabilities, or any other vulnerable groups. This is especially an issue because the experience of the second wave of the pandemic has provided an experiential learning that the Covid-19 virus is capable of mutation and now poses a threat to persons in this age group as well.”
In its affidavit earlier, the Centre had urged the court to trust the wisdom of the executive and leave policy decisions to it. On this, the bench said while separation of powers is a basic feature of the Constitution and policy-making is the sole domain of the executive, it “does not result in courts lacking jurisdiction in conducting a judicial review of these policies”.
Amid such situation, India has been trying to get more vaccines from abroad. India was hoping its Covid vaccine supplies will get a new push following External Affairs Minister S. Jaishankar’s visit to the US last week as New Delhi and Washington were able to “iron out” supply chain bottlenecks and issues around indemnity.
Jaishankar began his visit to the US last Monday with the primary focus of pushing ahead the health cooperation agenda both at the bilateral level as well as under the Quadrilateral Security Dialogue, or Quad, with the procurement of vaccines as the main issue.
Addressing a weekly briefing, MEA spokesperson Arindam Bagchi said, “The Government of India has been making all efforts to augment the availability of vaccines in India whether through enhanced production in the country or through supply from abroad we remain engaged with UN vaccine manufacturers like Pfizer, Moderna and Johnson and Johnson.” “As part of this effort, we are separately also engaged with US administration to ensure that components and raw materials for vaccine manufacturing production in India are readily available and the supply chain remains open,” Bagchi added.
Meanwhile, India has inched closer to procure US vaccines as India’s drug controller relaxes rule which stood as obstacles for these companies. On Wednesday, the Drug Controller General of India (DCGI) eased rules for importing COVID-19 vaccines, by exempting rules like mandatory bridging clinical trials and testing of each batch of imported vaccines at India’s Central Drug Laboratory (CDL) at Kasauli in Himachal Pradesh.
When asked will India be exporting vaccines in the future, MEA said India is right now focused on augmenting vaccine supply for domestic purpose and there is no question of exports at the moment
Meanwhile, amid discussions over possible legal protection to foreign vaccine manufacturers Pfizer and Moderna over their COVID-19 jabs in India, the Serum Institute of India (SII) has also sought protection against liabilities, sources said on Thursday. “Not just Serum Institute of India, all the vaccine companies should get indemnity protection against liabilities if foreign companies are granted the same,” sources added. Earlier last year, SII CEO Adar Poonawala had advocated for indemnity for all vaccine manufacturers.
Amid the nationwide COVID-19 vaccine crunch, US pharma giant Pfizer is seeking an indemnity bond that will exempt it from legal claims in case there are any adverse effects from the vaccine. NITI Aayog Member (Health) Dr VK Paul had said on May 27 that the government is still examining the American pharma company’s request for indemnity. “We are examining this request and will make a decision in the larger interest of people and on merit. This is under discussion and there is no decision as of now,” he had said.
No company in India’s vaccination history has ever paid indemnity and the government, which is the biggest user of vaccines, has also not done it, Dr Nirmal K Ganguly, former ICMR chief had said on Wednesday.
The Drugs Controller General of India (DCGI) had earlier exempted specific trials of COVID-19 vaccines that have been approved by some other international regulatory bodies. This is likely to clear the way of foreign COVID-19 vaccines such as Pfizer and Moderna.
Pfizer on Wednesday said it continues to remain engaged with the Indian government towards making its coronavirus vaccine available for use in the government immunisation programme.
Meanwhile, a day after COVID vaccine manufacturer Moderna allegedly refused to send vaccines directly to the Punjab government, Chief Minister Arvind Kejriwal on Monday informed that Pfizer and Moderna have refused to sell vaccines directly to the Delhi government and claimed they will only deal with the Central government.

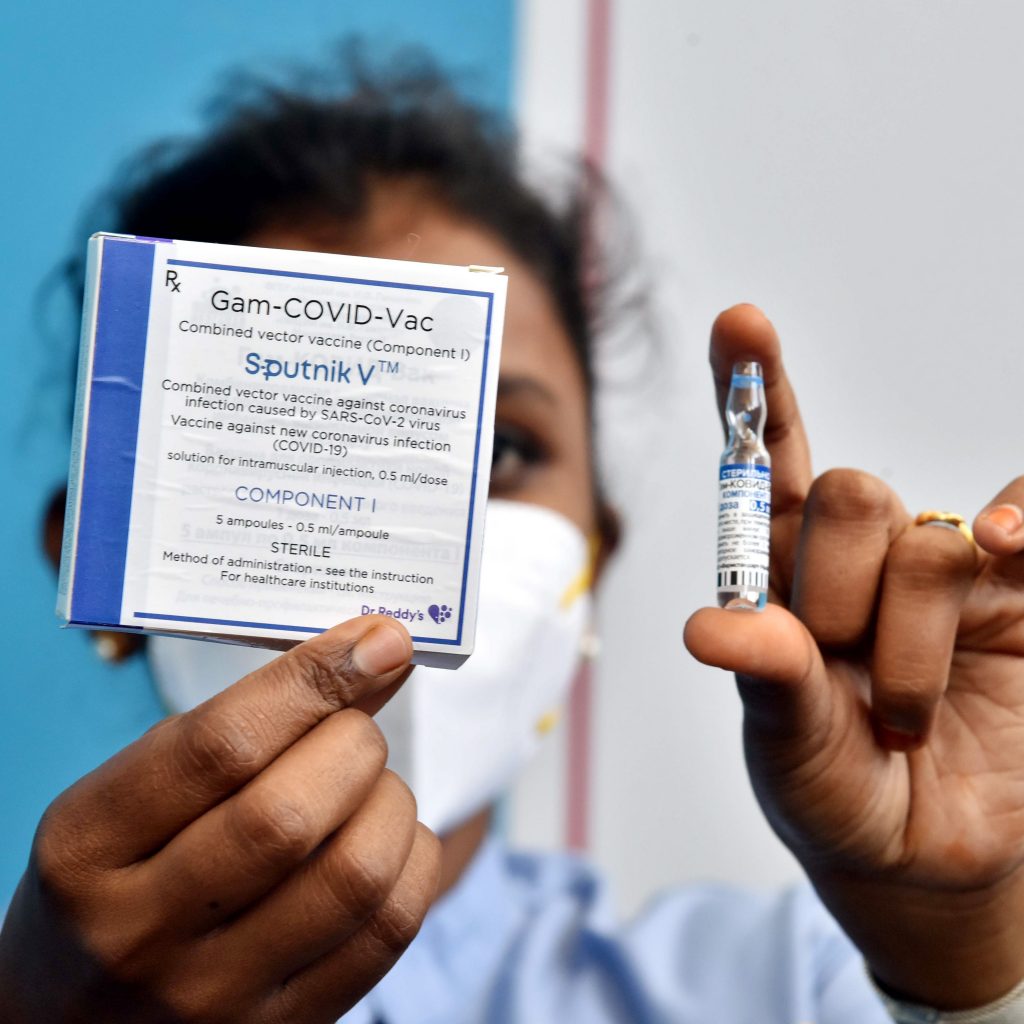
In another development, Indian vaccine manufacturing company Serum Institute of India (SII) has sought permission from the Drug Controller General of India (DCGI) for the production of the Russian vaccine Sputnik V in India. SII has applied for a test license to manufacture the COVID-19 vaccine.
Currently, Dr Reddy’s Lab is the Russian Direct Investment Fund (RDIF) partner, which markets the Sputnik V vaccine in India.
Meanwhile, as the downward trend of COVID-19 infections continues, India hopes that more countries would soon lift travel restrictions on Indians. In April when deadly second wave of COVID virus gripped India and country was reporting around four lakh cases a day, many countries had put India in the red list and some even imposed complete ban on entry of Indians the main reason behind these restrictions was spread of COVID variant B.1.617, which first emerged in India.
Now that steep COVID-19 curve has begun to bend downwards, India hopes that countries would open restrictions. Talking to reporters in virtual weekly briefing MEA spokesperson Arindam Bagchi said that some countries have started the process of easing restrictions.
“When COVID-19 second wave began, some countries imposed temporary travel restrictions on India. We expect that these restrictions should be lifted when situation normalizes. We saw some countries started this process of easing restrictions, other countries should do the same we expect,” he said.
UAE, Canada, Australia, UK , Oman, Nepal, Philippines, Maldives, Kuwait, Italy, France, Germany and New Zealand are among the countries which imposed restrictions on Indians. However, some countries had eased restrictions on travel from India. These include. Netherland, Russia, South Africa, Egypt and Turkey.